The group’s unique patterns of gold nanoparticles decorated a recent front cover of Journal of the American Chemical Society.
Chemistry Department group finds dazzling arrangements of gold nanoparticles
Xingchen Ye and his group in the Department of Chemistry demonstrated that gold nanoparticles could be induced to form unique crystals with promising properties. Their work was highlighted on the cover of the August 16th edition of the Journal of the American Chemical Society.
The extended solid materials that Ye’s group created have what are called tunable plasmonic and optical properties: characteristics that could make them highly-efficient metamaterials, sensors, and probes. The source of these materials’ versatility is their building blocks: tetrahedra (also known as triangular pyramids) 60 nanometers in edge length, less than one thousandth the thickness of a sheet of paper. Each tiny particle—called a nanotetrahedron—has four triangular faces with sharp edges.

Ye keeps dozens of four-sided dice in his office to help guests visualize them. “Each nanotetrahedron looks like this,” explained the assistant professor of chemistry, picking up a pink die. “But when they combine, there are a lot of structures you can build,” he said as he pulled out a model of fifteen dice arranged into three neat decahedra.
To better understand both the individual and group structure of the nanotetrahedra, Ye’s group collaborated with researchers from the University of Erlangen-Nuremberg in Germany. The investigators used a technique called FIB-SEM (Focused Ion Beam Scanning Electron Microscopy) to look into the interior of the crystal.
“Think about it as a very sharp knife that you can use to cut slices,” explained Ye. “After each slice you can take an image. From this little chunk, you can take thousands of images,” he said, highlighting a single nanotetrahedron.
This technique showed the researchers that, depending on which faces are in contact with each other, groups of nanotetrahedra assemble themselves into three types of surface tiles: squares, convex triangles and concave triangles. Thanks to attractive forces, these two conformations nestle into each other, forming neat, densely packed quasicrystals.
This quasicrystal structure was predicted more than a decade ago by computer simulations, but Ye’s work was the first to demonstrate it experimentally. Surprisingly, the researchers found one part of the structure that didn’t match up to computer models. The models had predicted an even balance between the concave and convex crystal forms, leading to an overall flat shape.
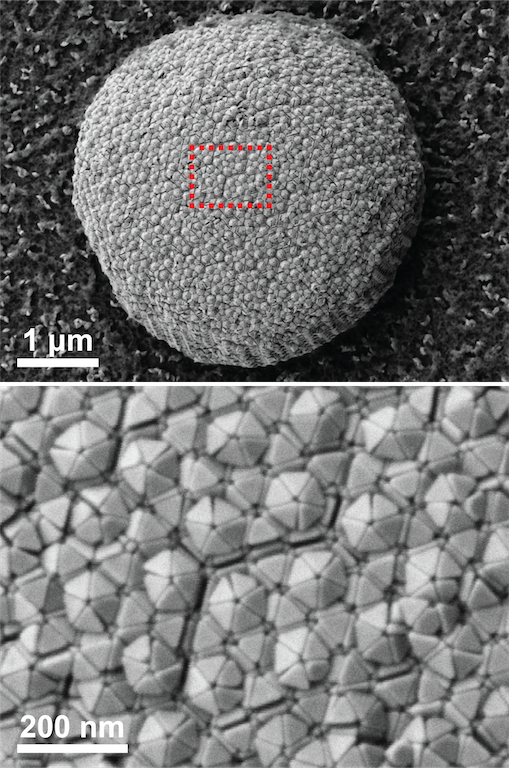
Ye’s group confirmed that, “if you have a very large surface crystal, it’s essentially flat,” but something odd happened in smaller crystals of nanotetrahedra. The crystals didn’t show the expected mix of concave and convex building blocks. Instead, “only the convex motif survived,” explained Ye. The computer models hadn’t predicted this because they assumed an infinite size of the material, but experiments showed that the convex-only form appears when size is limited.
Interestingly, the gold nanotetrahedra that Ye’s group created in this project had totally different organizations and properties from ones the group had reported in 2022. The difference between the 2022 and 2023 structures was that the former had rounded vertices while the latter had sharp ones. A change as small as a rounded tip at the nanoscale level dramatically changed the material’s properties.
By studying these unique nanoparticles, Ye hopes to help scientists better understand the possibilities of material structures, opening the door for functionalized materials for energy generation, catalysis, and beyond.
This work was funded by National Science Foundation grants DMR-2102526 and CBET-2223453.


